- Azoxystrobin
- Bitertanol
- Captan
- Carboxin
- Carbendazim
- Cymoxanil
- Cuprous Oxide
- Copper Hydroxide
- Cyproconazole
- Cyprodinil
- Dimethomorph
- Diniconazole
- Difenoconazole
- Epoxiconazole
- Fluazinam
- Fludioxonil
- Fosetyl-aluminium
- Hexaconazole
- Imazalil
- Iprodione
- Kasugamycin
- Mancozeb
- Metalaxyl
- Metalaxyl-M
- Penconazole
- Propiconazole
- Prochloraz
- Propineb
- Pyraclostrobin
- Thiophanate-methyl
- Tebuconazole
- Tetraconazole
- Tricyclazole
- Triadimenol
- Triadimefon
- Tridemorph
- Trifloxystrobin
Kasugamycin
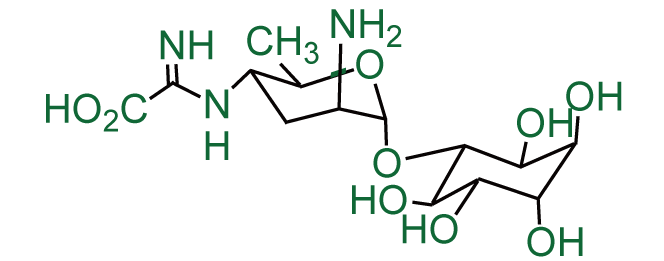 other name: 3-O-[2-amino-4-[(carboxyiminomethyl)amino]-2,3,4,6-tetradeoxy-a-D-arabino-hexopyranosyl]-D-chiro-inositol hydrochloride hydrate ; kasugamycin hydrochloride hydrate
other name: 3-O-[2-amino-4-[(carboxyiminomethyl)amino]-2,3,4,6-tetradeoxy-a-D-arabino-hexopyranosyl]-D-chiro-inositol hydrochloride hydrate ; kasugamycin hydrochloride hydrate
CAS No.: [19408-46-9]
Molecular Formula: C14H25N3O9
Kasugamycin is known as natural antibiotics, and kasugamycin inhibits proliferation of bacteria by tampering with their ability to make new proteins, the ribosome being the major target. Kasugamycin inhibits protein synthesis at the step of translation initiation. Kasugamycin inhibition is thought to occur by direct competition with initiator transfer RNA. Recent experiments suggest that kasugamycin indirectly induces dissociation of P-site-bound fMet-tRNAfMet from 30S subunits through perturbation of the mRNA, thereby interfering with translation initiation.
Kasugamycin specifically inhibits translation initiation of canonical but not of leaderless mRNA. For initiation on leaderless mRNA, the overlap between mRNA and kasugamycin is reduced and the binding of tRNA is further stabilized by the presence of the 50S subunit, minimizing Ksg efficacy. Kasugamycin also induces the formation of unusual 61S ribosomes in vivo, which are proficient in selectively translating leaderless mRNA. 61S particles are stable and are devoid of more than six proteins of the small subunit, including the functionally important proteins S1 and S12.
Kasugamycin has a unique site of activity from all other bactericides, which makes it effective against fire blight and streptomycin-resistant bacteria. Kasugamycin translocates in the leaves and is rainfast quickly after application.

APPLICATIONS
kasugamycin hydrochloride hydrate
Biochemistry
Protein synthesis inhibitor. Inhibits binding of Met-RNA to the mRNA-30S complex, thereby preventing amino acid incorporation.
Mode of action Systemic fungicide and bactericide with protective and curative action. Inhibits hyphal growth of Pyricularia oryzae on rice, preventing lesion development; comparatively weak inhibitory action to spore germination, appressoria formation on the plant surface or penetration into the epidermal cell. Rapidly taken up into plant tissue and translocated. In contrast, against Cladosporium fulvum on tomatoes, inhibition of sporulation is strong, but inhibition of hyphal growth is weak.
Uses
Control of fungal and bacterial diseases affecting rice, vegetables and fruit. On rice, it controls diseases caused by Pyricularia oryzae and Burkholderia glumae (bacterial grain rot), with ground and aerial applications, at 20-30 g/ha, and seedling diseases caused by various bacterial pathogens, at 0.3-0.6 g/box. In sugar beet, it controls Cercospora beticola, at 80-100 g/ha. Also used to control plant diseases in various crops: Pseudomonas syringae pv. lachrymans in cucumbers, at 30-60 g/ha; Colletotrichum lagenarium in melons and water melons; Cladosporium fulvum and Corynebacterium michiganense in tomatoes, at 20-40 g/ha; Mycovellosiella spp. in aubergines; Cercospora spp. in celery; Erwinia carotovora subsp. carotovora in potatoes, carrots and onions; Pseudomonas syringae pv. coronafaciens (halo blight) in kidney beans, at 40-60 g/ha; Xanthomonas spp. in paprika; Venturia sp. in apples; Pseudomonas marginalis pv. marginalis in kiwifruit; etc. Phytotoxicity Non-phytotoxic to rice, tomatoes, sugar beet, potatoes and other vegetables, but slight injury has been noted to peas, beans, soya beans, grapes, citrus, and apples.
Formulation types DP; GR; SL; UL; WP.
